The NMP program: Methods
Reef Survey
- Reef ecology
census
Goal:
Estimating the abundance, diversity, health, and community
structure at the three main reefs along the Israeli coast of the Gulf of Eilat.
The census includes stony and soft corals, and other invertebrates. It also
surveys the sea floor substrate as an estimate of the coral settlement
potential at each site.
Methods:
The reef community was surveyed at three locations, in each
at a number of depths (see table 1). The surveys take place annually in the
summer months.
|
Location
|
Name
|
Lat.
|
Long.
|
Depths (m)
|
|
Interuniversity Institute
|
IUI
|
34º55’.02
|
29º30’.07
|
5, 10, 15
|
|
Japanese
Gardens Nature Reserve
|
NR
|
34°55`.26
|
29°30`.33
|
5, 10, 20
|
|
Oil Terminal
|
KATZA
|
34°56`.04
|
29°31`.37
|
10, 20
|
Table 1: Locations and depths of the reef census surveys
Reef surveys employ the line transect method developed by Loya (1972). At each site a measuring tape extends from a
random starting point along the selected depth. The survey’s starting point is
a randomly derived distance from the measuring tape’s origin. From this point
ten-meter-long transects were performed in which everything under the tape is
noted with ±1 cm accuracy. Consecutive transects are separated by several
meters. Between 8 and 11 transects are performed at each survey location.
Corals and other invertebrates are identified to the Genus level, and the
substrate is noted as one of the following categories: Rock, Sand, Pebbles,
Dead Corals).
The size of coral colonies included in the survey is estimated
as belonging to one of the following categories: Small<5cm,
5<Medium<15cm, 15<Large<30cm, Huge>30cm. For each colony the
live fraction (in percent of the total colony area) is estimated. “Live” is
defined as the coral sections that are not dead, bleached, or covered by algae.
A site “Health Index” is defined as the average live fraction of all colonies
surveyed at the site. “Site” is defined as a given depth at a given location.
For each site the live cover, the distribution of substrate
types, the species diversity and abundance, the health index and the colony
size distribution are calculated.
Species diversity is calculated using the Shanon-Wiener index: 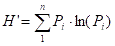
where Pi is the proportion of colonies of the species
(genus) i within the total species (genera)
surveyed. We use this index since it is the one used in Eilat by past workers (Loya, 1972) and so enables comparison of the NMP results
with previous data.
Statistical analyses are performed with the JMPIN 5.0.1
(SAS, NC USA) software.
In all graphs where error bars appear, they represent the
standard error.
Reef Flat: The reef flat at the Japanese Gardens is surveyed
as an independent site using the same line-transect methodology, without using
SCUBA gear.
- Photographs
of permanent locations
Goal:
Documenting and studying the dynamics of changes over the
years in the number of coral colonies, and their size and health, in fixed locations within the
reefs of Eilat.
Methods:
A photographic survey in fixed locations repeated annually
in the reefs of the northern beach, the Dekel Beach,
KATZAA, the nature reserve, the Interuniversity Institute, and Taba. At each site a permanent seating was attached to the
reef, on which a camera tripod can be connected so that the photograph will overlap
previous shots. The camera can be rotated to four directions thus providing
four photographs from each location. Five such seatings
were attached at each reef site. This setup ideally provides 20 photographs
from each reef, 120 photos in all. In reality some seatings
may be abandoned due to movement of the seating, or coral growth that obstructs
the view. The photo-survey takes place every year at the end of summer.
Every photograph is digitized using GIS, and all corals and
sessile invertebrates are counted, and identified as possible. Where pictures
do not overlap completely, all data is measured and stored but only colonies
appearing in consequent photos are analyzed for area change. Quantitative
analyses of area change in consecutive photo sets include “growth” - colonies
appearing on both sets being compared, death and recruitment of new colonies,
and the total coral area change within the compared frame – “area change”.
Corals are identified to the genus or species level as possible, and are classified
as “branching” or “non-branching” stony corals, soft corals, and hydrocorals.
- Coral
ecology census in the nature reserve lagoon
Goal:
Estimating the coral abundance, diversity, health, and
community structure at the southern part of the lagoon of the marine nature
reserve of Eilat. The census includes stony and soft corals, and other
invertebrates. It also surveys the sea floor substrate as an estimate of the
coral settlement potential at each site.
Methods:
Due to the relative scarcity of corals in the lagoon we use
a systematic, rather than random, survey array. Sampling units are 1m2
quadrates placed along a measuring tape. The measuring tape extends
perpendicular to the shore and the first quadrate is placed 9 meters from the
shoreline that is marked by the occurrence of algae on the outcrops of beachrock. Subsequent quadrates are placed at five meter
intervals into the lagoon, but no more than six quadrates per line. The first
survey line is at the northern end of the closed section of the reserve, and
subsequent lines are 25 meters apart to the south, ending at the underwater
observatory where the lagoon becomes indistinct. In all the survey covers
around 400 meters along the shore, and up to 35m of the lagoon width. Corals
are identified to the genus or species level, counted, and their size is
estimated. An estimate of the percent coral cover within each quadrate is
given, as well as an estimate of the distribution of substrate types. Only
corals whose center is within the quadrate are counted. Corals on knolls and
the back reef (where reached) are not counted.
- Census
of other (motile) invertebrates
Goal:
Estimating the abundance of the major motile invertebrates
in the reefs at the southern part of the Israeli coast
Methods:
This survey takes place after dark since many of the
organisms sought are nocturnal. Two reef sites are surveyed: the IUI and the
closed part of the marine nature reserve, at 5 and 10 meters depth. The survey
begins an hour after dark with deployment of a 100 m long measuring tape along
the chosen depth (isobath). Quadrates of 1m2 are placed along the
tape five meters apart from a random starting point. All motile invertebrates
within the quadrates are counted.
- Macro
algae growth in the reef
Goal:
Estimating the rate and amount of benthic algae settlement,
and the effects of grazing in the reef.
Methods:
Six sets of 10x10 cm PVC plates were placed arbitrarily at a
depth of 7-10 meters in the IUI reef and at three depths, 5, 10 and 20 meters,
at the Japanese Gardens coral reef nature reserve. Each set contains a plate
that is exposed to grazing and one that is protected from grazers by a cage of
netting with 1cm2 holes. The plates provide a substrate for benthic algae
settlement, imitating exposed areas in the reef. Once a month three sets are
retrieved and replaced by a clean set, giving each set a two-month period
underwater.
In the lab the algae is scraped off the plates and filtered
through a glass fiber GF/F filter. The filter is then placed in an
acetone-methanol solution (50:50 by volume) to extract photosynthetic pigments
that will be used to estimate the algae mass. Light absorption in the
photosynthetic pigment wavelength (E647, E630, E664) is measured on a
spectrophotometer in is used for calculation of the amount of chlorophyll-a,
the main photosynthetic pigment of these algae, through the formula:
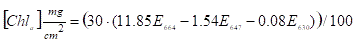
Due to local variability in algae settlement each monthly
estimate is the average of the three sets retrieved that month.
The potential mass of algae settlement in the reef over a
two-month period is estimated from the plates that are protected from grazing
by a mash cage. This potential is largely determined by the availability of
nutrients. The exposed plates simulate the true algae settlement in the reef
over a two month period, and provide an indirect estimate of the grazing activity
in the reef.
6. Coral settlement at the Japanese Gardens coral reef nature
reserve
Goal:
Following seasonal and inter-annual changes in the influx of
potential coral settlers through repeated season-long examination of ceramic
settlement plates.
Method:
Three arrays of unglazed terracotta plates are placed each
April at 10 meters depth at the IUI and Japanese Gardens reefs. Each array
holds 12 plates and every two months three plates are removed from each array
and are immediately examined under a reflected light microscope (binocular)
with and without UV light. Every coral spat is counted, the number of polyps in
each spat is noted and when possible the coral genus is identified. The last
plates are removed in November so that the period April-November, spanning the
entire reproduction season is covered.
The coastal environments
1. Coastal
waters
Goal:
Monitor the chemical, physical, and biological variables in
the water column near the shore, and recognize long-term trends and sources of
contamination that may affect the reef.
Method:
Water samples and physical measurements are collected
monthly in seven stations along the Israeli coast, and one open sea station,
(see table 2). The survey is conducted from a small boat in the late morning
hours and takes about two and a half hours. Surface water (1-2 meters depth) is
collected in 5 liter GOFLO bottles for analyses in the laboratory. Surface
temperature is measured with a high precision mercury thermometer, and
visibility in the water is estimated with a Secchi Disk (a standard white disk
is lowered from the boat until it cannot be seen – the “Secchi Depth”.
|
name
|
location
|
longitude GPS (N)
|
latitude GPS (E)
|
|
FF
|
Northern beach,
near fish farms
|
29o 32’.25
|
34o 55’.75
|
|
NB
|
Northern beach, off
the Dan Hotel
|
29o 32’.94
|
34o 58’.23
|
|
N
|
Southern end of
navy base, 50m east of the Meridien Hotel water outlet
|
29o 32’.55
|
34o 57’.36
|
|
PT
|
The phosphate
terminal at the port
|
29o 31’.76
|
34o 57’.09
|
|
WPC
|
The water pollution
control station
|
29o 30’.87
|
34o 56’.54
|
|
NR
|
Japanese Gardens,
nature reserve, close to observatory
|
29o 30’.33
|
34o 55’.78
|
|
T
|
Off the Taba border control
|
29o 29’.41
|
34o 54’.24
|
|
OS
|
Open Sea, off IUI
at 350m depth
|
29o 30’.11
|
34o 56’.52
|
Table 2: Locations and names of stations visited in the
coastal waters surveys
Upon return to the lab the following analyses are performed:
Dissolved oxygen: the dissolved oxygen concentration
is determined by Winkler’s reaction. Samples are fixed by addition of two
reagents, MnSO4 and KI+NaOH, and titrated
with 0.1N Na2S2O3.
Titration is done with Titrino 702 SM automatic
titrator (Metrohm, Switzerland).
pH: measured at a constant temperature of 25˚C
with a pHC2401-7 combined pH electrode and PHM 93 pH-meter (Radiometer,
Copenhagen).
Salinity: measured with a Minisal
2100 salinity meter (AGE Instruments, Canada).
Nutrients: NO2, NO3, SiO2,
PO4 measured with QuikChem 8000 flow
injection analyzer (Lachat Instruments, Milwaukee,
USA). This is a color reaction where each nutrient reacts with a specific
reagent producing a specific color complex that is read by the instrument’s
spectrophotometer.
Alkalinity: determined by Gran titration with 0.05N
HCl, on a DL67 automatic titrator and DG111 combined pH electrode (Mettler
Toledo, Switserland).
Chlorophyll: Water is filtered on a GF/F filter that
is placed for 24 hours in 90% acetone at 4˚C in the dark, after which the
concentration of chlorophyll-a and phaeophytin is
measured on a 10-AU Fluorometer (Turner Designs, California USA). Phaeophytin is measured after addition of 1N HCl to the
acetone extract.
Ammonium: measured with a DyNA
QuantTM 2000 fluorometer (Hoefer)
following 3 hours of incubation in the dark with a color reagent containing orthophthaldialdehyde.
Phytoplankton cell counts: performed on a Becton
Dickinson FACScan flow cytometer that sorts cells
according to size and pigment content. The (water) sample flows across a laser
beam that ineracts with each cell separately and is
read by sensors that measure the wavelength absorption and ray diffraction by
the cell providing information on cell size and pigment content. The NMP tracks
eukaryotic cells, bacteria, and the cyanobacterium Synechococcus.
2. Sandy
sea floor
Goal:
Following changes to the infauna living in the top-most
sedimentary surface, as indicators for changes in habitat conditions.
Method:
Muddy sediment is collected at fixed locations at 20 meters
depth at the northern and southern shores of Eilat, by divers using fixed
volume collection tubes. Samples are collected for characterization of particle
size distribution, benthic foraminifera taxonomy and other
infauna taxonomy. Triplicates are sampled for each measurement.
Samples for particle size distribution are oven-dried at 100
degrees centigrade for 24 hours and then weighed. The smallest fraction is
washed away through a 63 micrometer sieve and the samples then dried and
weighed again. The remainder dry sediment is placed in a sieve stack of 63,
125, 250, 500, 1000, 2000 micrometers and is shaken for ten minutes. Each
fraction is weighed and the relative weight of every fraction is calculated.
Samples for infauna taxonomy are fixed with 4% formaldehyde,
Sodium-tetraborat
buffer at pH 8.2 for 24 hours, then washed through 250 and 500 micrometers seives and each fraction is incubated with a 70% ethanol
and Rose Bengal (1 gr/liter) solution for a week. Samples are then washed and
placed in 70% ethanol until examination under a microscope (binocular).
Individuals are identified and counted and the results are normalized to 10 cm2
surface area. Representative specimens are photographed and preserved.
Samples for foraminifera taxonomy are collected from the top
1 cm of the sediments and are then incubated in an ethanol-Rose Bengal (2
gr/liter) solution for 14 days. Samples are than dried, weighed and split to
sub-samples for identification and counting.
3. Seagrass density
Goal: Assessing the changes in seagrass density over sandy
bottom at the northern and southern coasts of Eilat.
Method:
Seagrass density is estimated every summer for
well-developed meadows at the northern and southern shores of Eilat, at three
depths (10, 15, 20 meters). At each seagrass depth a shore-parallel transect,
50 meters long is placed on the seafloor and a 1X1 meter frame is placed and
photographed at 5 meter intervals along the transect. Using the CPCe software 100 random points are generated on each photo
and the presence/absence of seagrass at each point is noted. The cover density
at each photo is estimated by the relative occurrence at 100 points, and the
site-depth average is calculated.
The open sea
1.
Seawater properties
Goal:
Monitor the chemical, physical, and biological variables in
the open sea water column, and recognize long-term trends in water properties.
Method:
Water samples and physical measurements are collected
monthly at three stations along a north-south transect parallel to the
Israeli-Jordanian border. The northern most station is near the Fish Farms at
the northeastern end of the Israeli coast, at approximately 55m water depth.
The southern most station is Station A, at the triple border of
Israel-Jordan-Egypt, where water depth exceeds 700m. Half way between these is
the Open Sea station, some 4km south of the Fish Farms, at 400m water depth.
The survey is conducted from a ship using a rosette of 11 10-liter GoFlo sampling bottles and a CTD. The CTD collects
continuous physical data while water is sampled by remotely closing the
sampling bottles at the desired depths. The deep water is sampled at 50m intervals
while the shallow water, from the base of the thermocline and up, is sampled at
20m intervals. The deepest sample at each station is from just above the sea
floor.
A continuous vertical profile of temperature, salinity,
pressure, and fluorescence in the water column is measured in-situ with a
Sea-Bird Electronics CTD. Sampled water is collected in dedicated bottles for
analyses of dissolved oxygen concentration (DO), pH, alkalinity (AT), nutrient
concentrations (PO4-3, SiO2, NO3-1, NO2-1), salinity, Chlorophyll-a, and
phytoplankton cell counts (FACS). Filtration for chlorophyll-a and cell counts,
and samples fixation is done on-board. Analyses are performed at the laboratory
using the methods outlined above (see section “Coastal waters”).
According to convention and to better fit the water samples
(collected on the way up) the CTD data used in analyses are the up-cast data.
To reduce noise, CTD data is averaged into discrete 1 db (~1m) intervals.
Salinity and fluorescence data are further treated by wden.m
(wavelet denoising) from the Matlab wavelet toolbox,
to reduce white noise.
2.
Phytoplankton cell counts
Goal:
Characterizing the phytoplankton community (Synechococcus,
Prochlorococcus, pico-eukariots and
heterotrophic bacteria.
Method:
During each monthly cruise, water
samples are collected from every sampled depth (see above) into 5ml vials and
are immediately fixed in glutaraldehyde and placed in -80 degrees freezer. The
samples are sorted and counted with a flow cytometer at the biological
infrastructure facility of the Technion.
3.
Zooplakton
biomass
Goal:
Following seasonal and inter-annual changes in zooplankton
abundance in the upper waters away from the shore.
Method:
Each month three net tows are done across from the
Underwater Observatory over at least 300 meters depth. During each tow a double
"Bongo" net, 200 micrometers mash size and equipped with a TSK flow
meter, is lowered slowly to 100 meters depth an raised again all while the boat
is slowly moving forward. Each tow takes approximately 7-10 minutes. Samples
are filtered immediately after collection over a stacked array of 200, 500,
1000 micrometer filters. Each fraction is then filtered on a pre-burned (450
degrees for 4 hours) GF/F filter. The filters are oven-fried at 60 degrees for
at least three days and weighed. The filters are then placed at 450 degrees for
4 hours and weighed again. The ash-free dry weight (AFDW) is calculated from
the weight difference before and after burning.
Continuous measurements
1. Chlorophyll-a adjacent to the reef front
Goal:
Determination of long term trends and changes in
phytoplankton density near the reef
Methods:
Daily measurement of chlorophyll-a concentration in
duplicate water samples collected at a fixed location off the pier of the
Underwater Observatory near the fore-reef. Surface water is collected into
300ml bottles. It is then filtered on a GF/F and extracted placed in 10ml
Buffered Acetone (90%) for 24 hours at 4˚C. The concentration of
chlorophyll-a is measured on a TD-700 Turner Designs fluorometer, calibrated
daily with a standard that is measured along with the samples.
2. Sea surface temperatures at the nature reserve
Goal:
Long term monitoring of the sea surface temperature
Method:
The sea surface temperatured is
measured at a fixed location off the pier of the Underwater Observatory, using
a high resolution (0.1 degree) mercury thermometer.
3. Current speed and direction
Goal:
Long term monitoring of currents, speed and direction, near
the shore of the IUI
Method:
An acoustic Doppler Current Profiler (ADCP), 600 kHz, is
located on the sea floor off the IUI at 30 meters depth. The ADCP’s beam is
directed upward toward the surface and measures the current at 1 meter intervals
between 3 and 27 meters, every 10 minutes. Data analysis includes the removal
of discrete offset measurements (>2sd).
4. Sea water temperature and sea level
Goal:
Long term continuous monitoring of sea surface temperature
at the IUI, and of fluctuation of the sea level
Method:
Sensors for water pressure and temperature (Campbell Scientific, models CS408 and 108
respectively) were installed beneath the IUI research pier, fixed to its main
supporting pole. The sensors are connected to a data-logger where measurements
are automatically stored (see following section, ‘Meteorological
measurements’). Water temperature readings are taken every second and are
averaged over a ten-minute period. Water level measurements are recorded every
minute and averaged over a ten-minute period. The measurements are stored in
the NMP space on the IUI server.
5. Meteorological measurements
Goal:
Providing continuous measurements of meteorological
conditions over the sea surface at the IUI, for the support of scientific research
and to compliment oceanic measurements
Method:
A suite of meteorological sensors was installed on the IUI
pier, and measurements are automatically recorded on a dedicated data-logger (Campbell Scientific, model CR1000)